Of the Following Which Element Has the Most Protons
Which of the following elements would be an isotope of calcium. Therefore chlorine is the element that has 17 protons.

Periodic Table Quiz Education Com Physical Science Middle School Science Worksheets Teaching Chemistry
A certain ion X contains 24 electrons and 49 neutrons.
. Radon Rn has the most protons of any element in Group 0 18with 86 protons. Hence the atomic number of the element will be 17. C electron shells that surround the nucleus.
The model shows values corresponding to a mystery element element X on the periodic table. This element is in the same family as Br but has fewer protons than Mg. The symbol for nitrogen is.
The mass number of the ion is 15 16 31. An atom with 21 protons 20 neutrons and 21 electrons. The number of protons is the same as the atomic number.
Which of the following elements has a symbol that does not come from the. Which of the following elements has the most protons in its nucleus. The number of protons in a neutral atom is balanced by an equal number of a orbital electrons.
Which of the following elements would be an isotope of calcium. 112 rows Rhenium has 75 protons 111 neutrons and 75 electrons. 8 protons and 8 neutrons Hydrogen.
1 proton and no neutrons crown. Also the atomic number of Cl 17. Which of the following elements has the most mass.
539 x The elements symbol is It has protons It has neutrons c. 2x The elements symbol is It has protons It has neutrons d. Hence the element is Chlorine.
The symbol for lead is. D 17 protons 20 neutrons. These are the most stable and least reactive elements due to having full valence shells the outer shell has the max number of electrons two for helium eight for the rest.
Element Atomic Number Oxygen 8 Lithium 3 Hydrogen 1 Carbon 6 Which element has the most protons in the nucleus. An ion with 5 protons 6 neutrons and a charge of 3 has an atomic number of a. 2 protons and 2 neutrons Nitrogen.
7 protons and 7 neutrons Oxygen. The number of electrons in an atom of element 17. By referring to a periodic table or table of elements we see that phosphorus symbol P has an atomic number of 15.
X The elements symbol is It has protons It has neutrons b. An atom with 21 protons 21 neutrons and 21 electrons 3. Which of these has the greatest number of protons in its nucleus.
Which of the following elements has the highest atomic mass. What is the mass number of an atom which contains 28 protons 28 electrons and 34 neutrons. Neon is the fourth most abundant element in the universe according to the Jefferson Laboratory.
This number is the atomic number. Thus each atom has 15 protons. Each element is identified by the number of protons in its atoms.
Table for element 15 phosphorus. A 20 protons 17 neutrons B 17 protons 37 neutrons C 17 protons 20 neutrons D 37 protons 17 neutrons E none of the above Answer. B neutrons in the nucleus.
Choose the correct terms to complete the sentences. PD Na Pd p. There are 118 elements on the periodic table.
Thus as per the relation. Identify the following elements. Give the number of protons and neutrons.
This element is in the family with the most reactive nonmetals and has 4 energy levels. The most common form of calcium has 20 protons 20 neutrons and 20 electrons. The atom with 69 neutrons and 50 protons has a mass number of.
Each element has a symbol which is one or two letters. The symbol Ga stands for the element. D none of the above.
An atom with 20 protons 20 neutrons and 18 electrons b. Radon Rn has the most protons of any element in Group 0 18 with 86 protons. Because the ion has 15 protons and 18 electrons three more electrons than protons its net charge is 3-.
A lead b uranium c hydrogen d iron e all have the same mass. The number of protons in the nucleus of the atom is equal to the atomic number Z. What is the mass number of this ion.
There are 5 protons so the atomic number is also 5. Use the following table to answer the question. An atom with 21 protons 20 neutrons and 21 electrons c.
However if or when Ununoctium is officially confirmed that will become the element with most protons in that. The ----- is the total amount of protons in the nucleus of an atom which is represented by the number ----- in the model. List of the Elements.
Oxygen Lithium Hydrogen Carbon. As atomic number number of protons 17. An atom with 18 protons 20 neutrons and 18 electrons.
The number of protons in the atom 17. However if or when Ununoctium is officiallyconfirmed that will become the element with most protons in thatfamily. The periodic table lists the elements in order of increasing atomic number.
An atom with 20 protons 21 neutrons and 20 electrons 2. Since Cl - 37 has mass number 37. 166 68 The elements symbol is It has protons It has neutrons.
The most common form of calcium has 20 protons 20 neutrons and 20 electrons.

Thorium By Carlos Clarivan Atomic Structure Thorium Electron Configuration

Them Unununuium Atoms Most Protons On Periodic Table Unununium Google Search Second Most Bohr Model Element Project Atom
Which Element Has The Same Number Of Protons And Neutrons In Their Atoms Quora

Elements Trading Cards Chemistry Worksheets Middle School Science Teacher Teaching Chemistry

The Vast Majority Of The Elements In The Periodic Table Are Metals Metal Show A Wide Range Of Chemical And Carbon Periodic Table Periodic Table Matter Science

Hydrogen Elements Number Of Protons 1 Neutrons 0 Electrons 1 Ppt Video Online Download
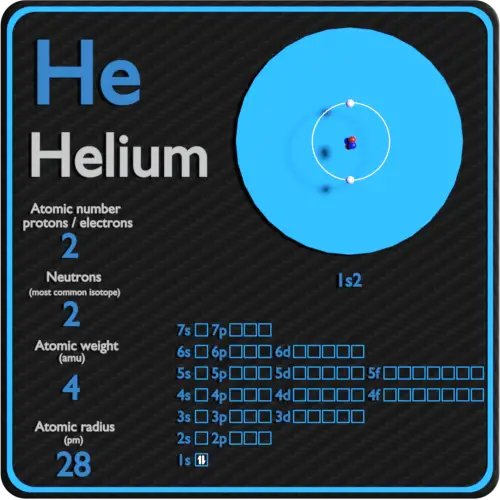
Helium Protons Neutrons Electrons Electron Configuration
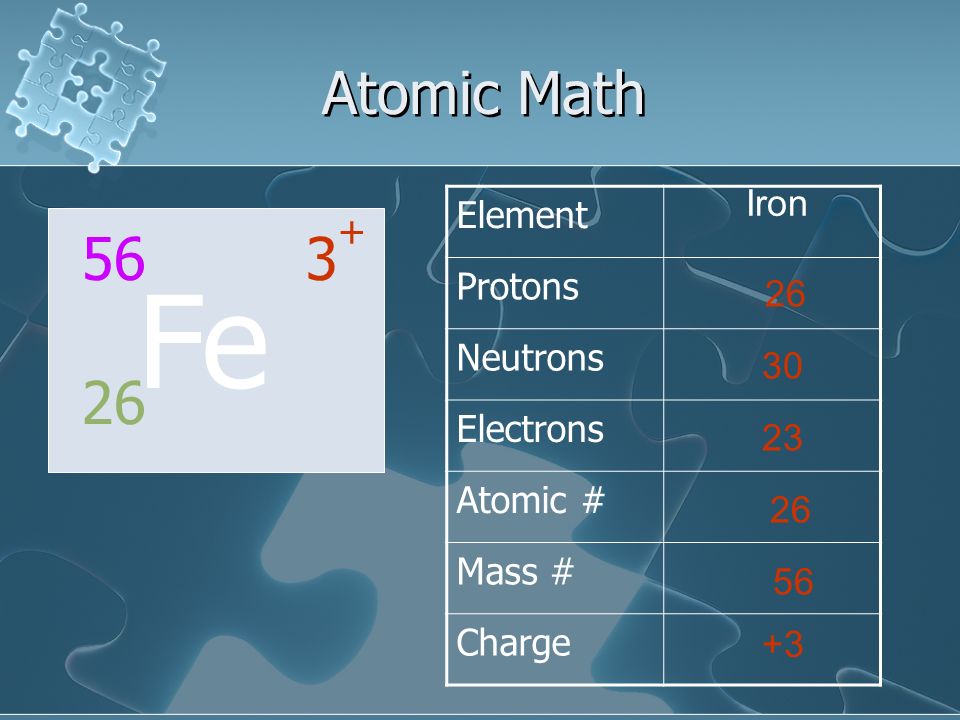
Elements Elements Elements Ppt Video Online Download

Worldofchemicals On Twitter Atomic Theory Atom Diagram Atom Model
No comments for "Of the Following Which Element Has the Most Protons"
Post a Comment